
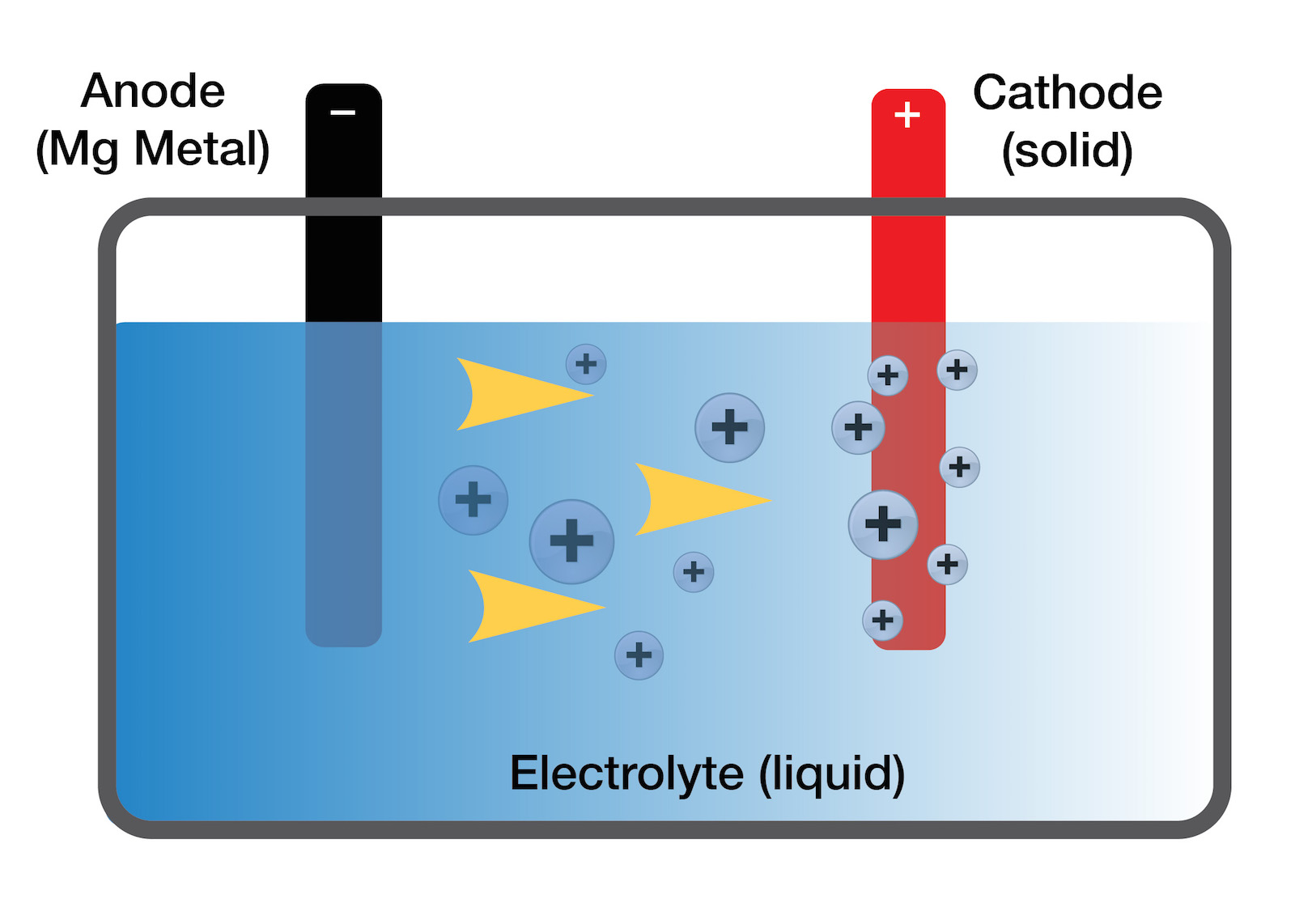
Consequently, the use of Al as anode in metal–air batteries has long attracted attention because of its high theoretical ampere hour capacity and overall specific energy. Because of its lower reactivity, easier handling, and greater safety, such an Al-based battery may offer significant cost savings and safety improvements over Li ion batteries. Its relatively low atomic weight of 26.98 and trivalent state confer it a gram-equivalent weight of 8.99 and an electrochemical equivalence of 2.98 A h g −1, as compared with 3.86 A h g −1 for lithium. An aluminum-based redox couple, which involves a three-electron transfer that occurs during the electrochemical charge/discharge reactions, provides a storage capacity that rivals that of the single-electron lithium-ion battery. Meanwhile, aluminum (Al) is inexpensive, safe, and is the third most abundant element in the Earth's crust. In addition, lithium is very sensitive to ambient conditions, such as humidity and oxygen, and it is a scarce natural resource in some regions. However, the rechargeability, safety, and cost of these batteries make them difficult to commercialize. 12–15 Among various metal–air batteries, alkaline metal–air batteries, especially lithium–oxygen, have been intensively investigated because of their high specific energy density of up to 5200 W h kg −1. 10,11 Recently, metal–air batteries have become a promising power source because of their high theoretical energy density and their use of atmospheric oxygen as fuel. 5,6 In this regard, many post-lithium-ion technologies, including Li–S, 7 Na ion, 8 all-solid-state Li ion batteries, 9 and metal–air batteries, have been proposed and actively studied. Unfortunately, their limitations of high cost, insufficient energy density, and unsatisfactory safety have prevented their large-scale applications in the automobile industry, especially for extended-range EVs.
CATHODE REACTION OF ALUMINUM AIR BATTERY PORTABLE
1–4 To date, Li ion batteries are the most successful energy-storage solution they have been widely used in both portable electronics and electric vehicles (EVs) since the first report was made on them in 1991.

Introduction Advanced materials that permit the efficient harvesting, storage, and utilization of renewable energy are at the heart of ongoing research in the energy field. Even though we did not detect byproducts, we observed NaCl and NH 4Cl phases on the air cathode, and they did not hinder the electrochemical reaction. When TiN was used as an air cathode material, byproducts of the aluminum–air battery such as Al(OH) 3 and Al 2O 3 were not detected on either the Al anode nor the air cathode.

The prepared battery demonstrated a capacity smaller than the theoretical value although we observed stable electrochemical reactions. In order to create a rechargeable aluminum (Al)–air battery, an aluminum–air battery with a deep eutectic solvent-based solid electrolyte was prepared.


 0 kommentar(er)
0 kommentar(er)
